In September we launched new services for all EGA users, including a new version of the former Submitter Portal. Our main objective with this Portal is to transform the metadata submission in a simplified and user-friendly method.
Although it is possible to find documentation about the Submitter Portal on our website, we wanted to highlight the new features in this article. Enjoy!
Credentials: who needs to create a new user to access the Submitter Portal and who doesn't?
Users with an old submission account (ega-box) can already access the new Submitter Portal.
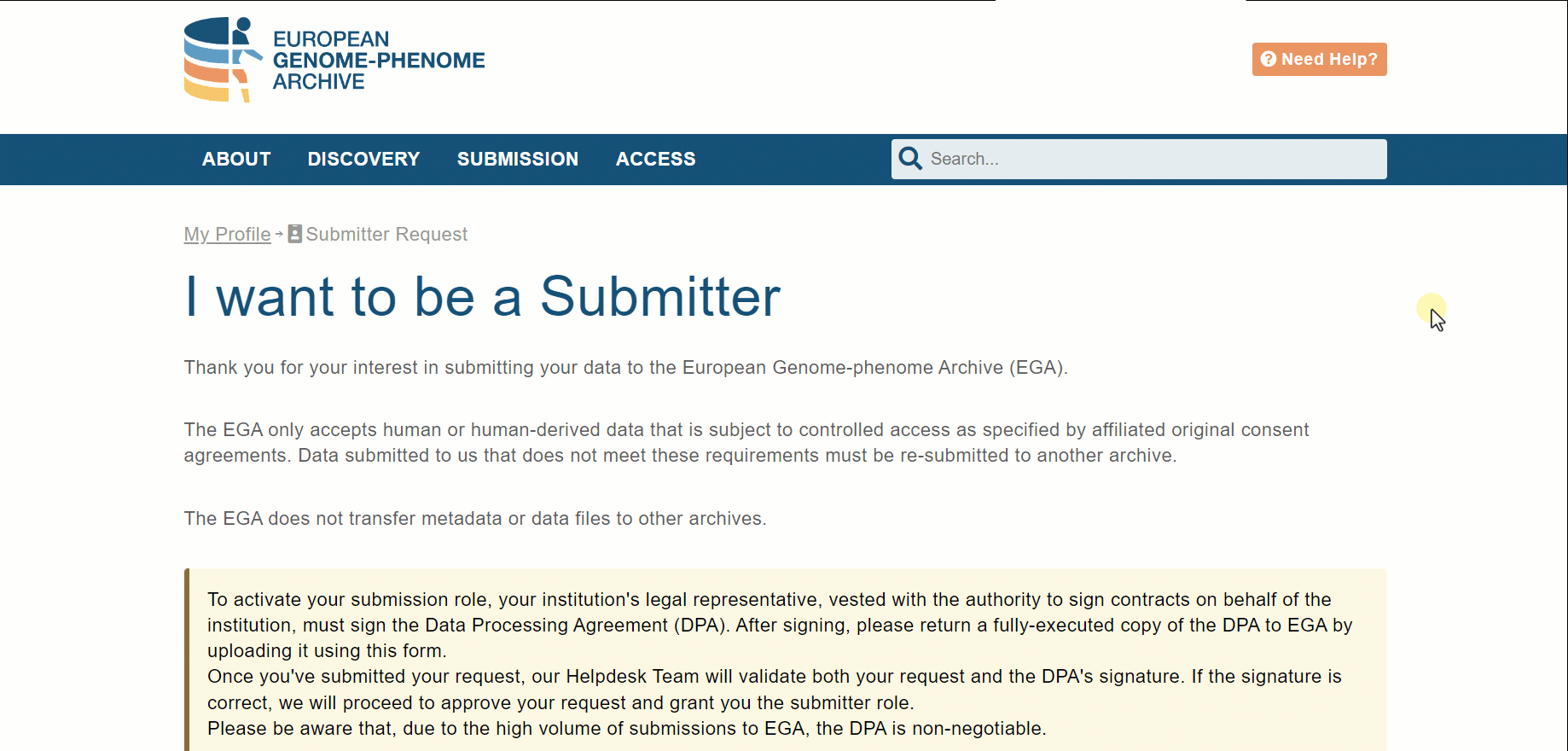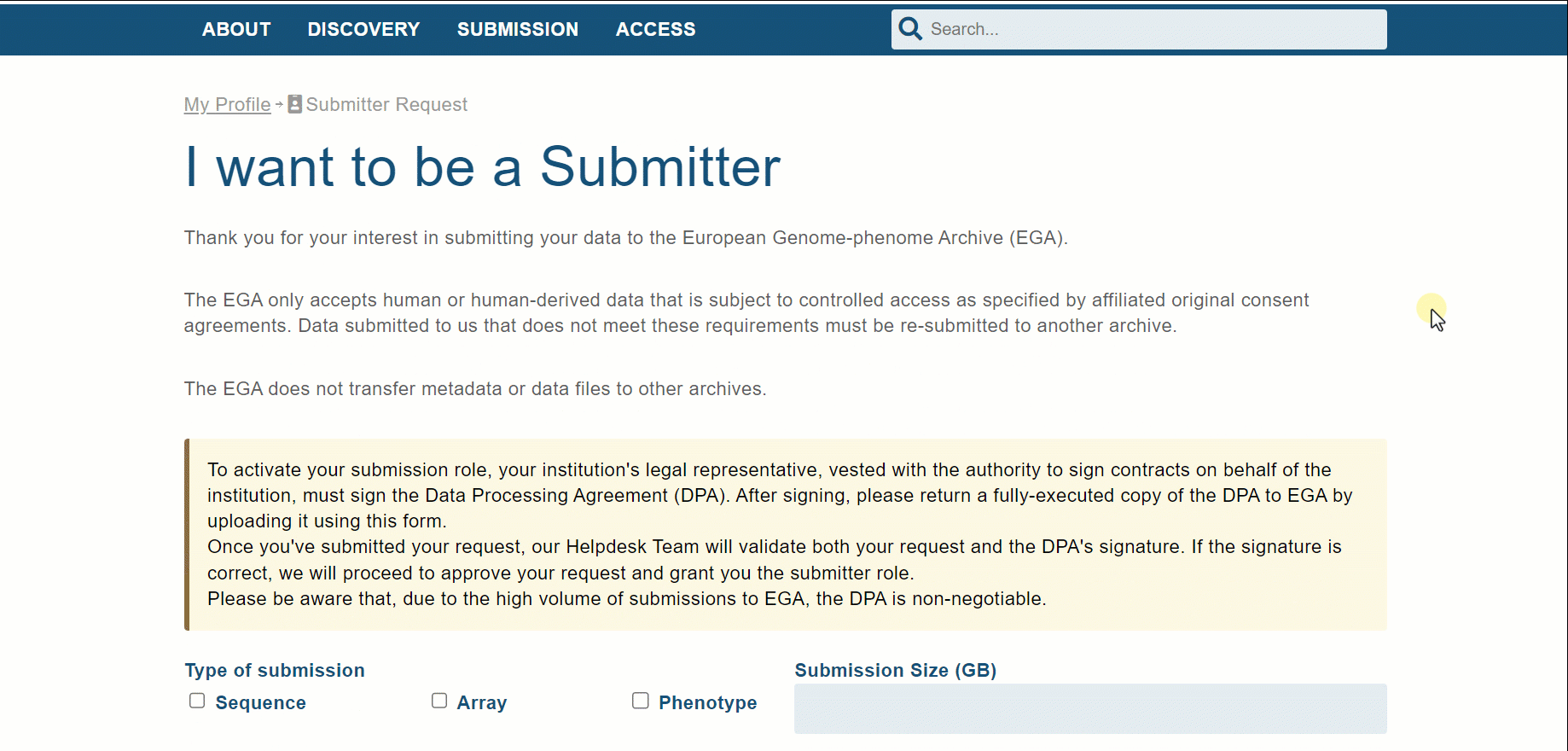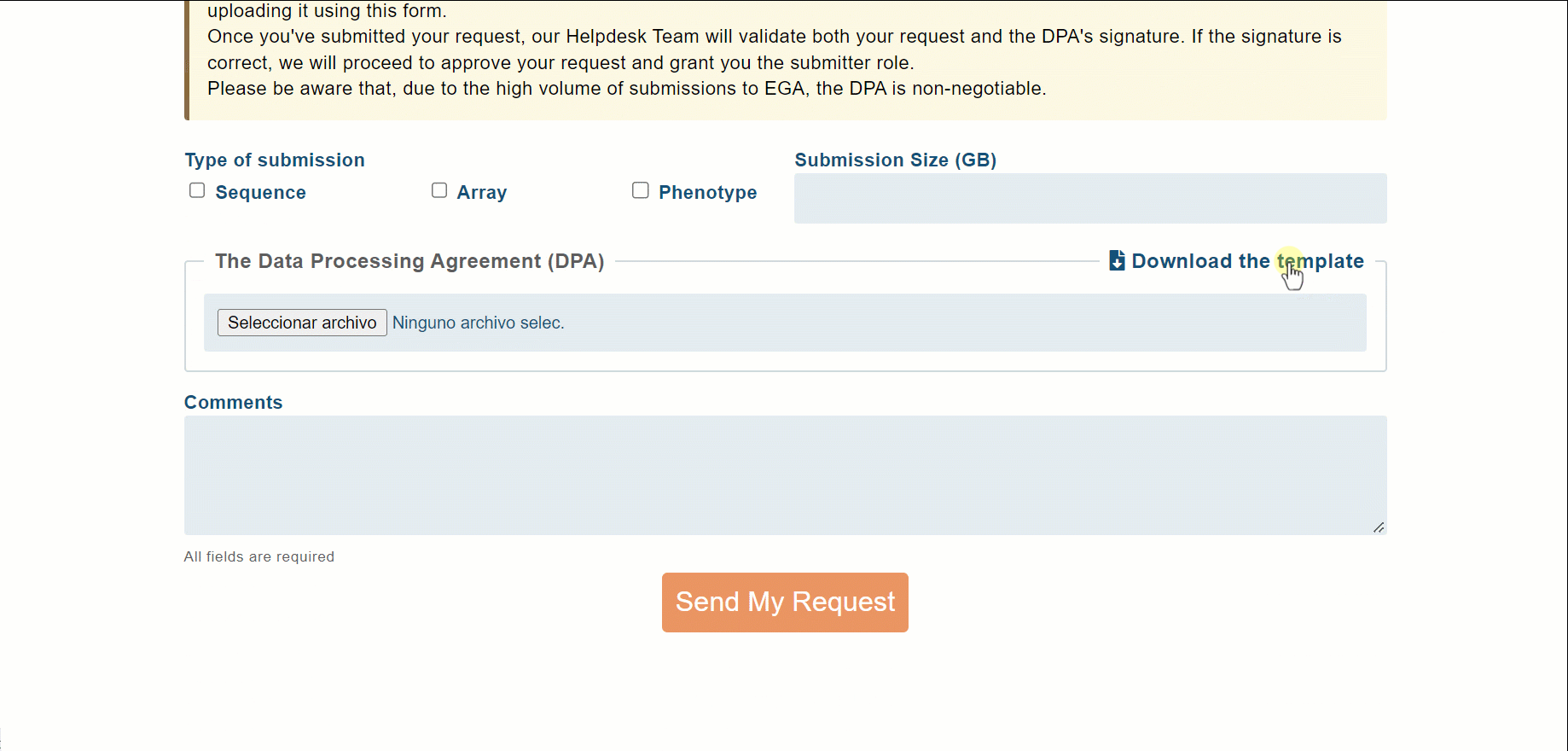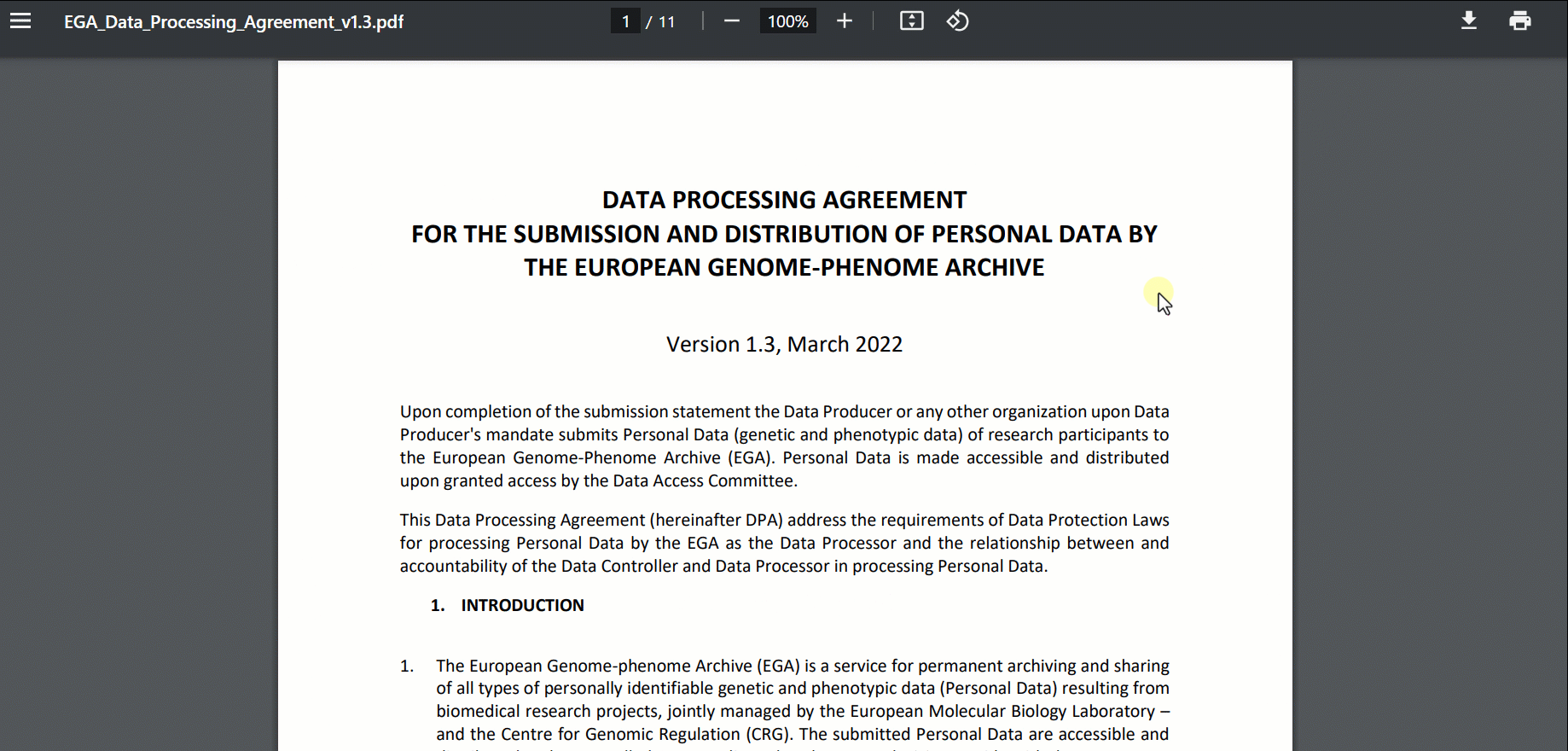
If it is the first time that you want to access, it is time to request a submitter role linked to the EGA User. In the form, it is mandatory to explain which type of data you want to deposit, the size of your submission, add any comment you’d like us to read, and manage the EGA Data Processing Agreement (DPA) document.
This document should be checked by the legal department of your institution and signed by a person with authority to sign contracts on behalf of the institution. Note that we provide a DPA template for new Users; it can be found and downloaded in the Submitter Request section.
With the DPA signed and the Submitter Request sent, it is time to wait for our Helpdesk team to validate the request.
Metadata submission now can be collaborative
It is possible to add collaborator(s) to your submissions. Only registered EGA Users will appear on the search bar.
When adding a collaborator, it is mandatory to define the permission granted:
- Read only: collaborators with this permission can check if the information of the submission is correct but cannot modify anything.
- Read & write: collaborators with this label can register new objects and modify the registered ones.
We wanted to save time for submitters that usually rely on the same collaborators. For that, we have implemented the possibility of adding the same collaborators from previous submissions; all you need to do is to search the submission, and the exact set of EGA users will have access to your submission.

You can check the collaborators and their permissions at any point of the submission by going to the collaborators section. Even adding more people to complete the submission is allowed by following the same steps: looking for the EGA User and defining the permission granted (please, note that during the submission it is not possible to add collaborators from previous submissions).
Use external links to complement the study information
When filling the information about the study we recommend users to enrich the submission with complementary fields such as PubMeds IDs, custom tags and external links.
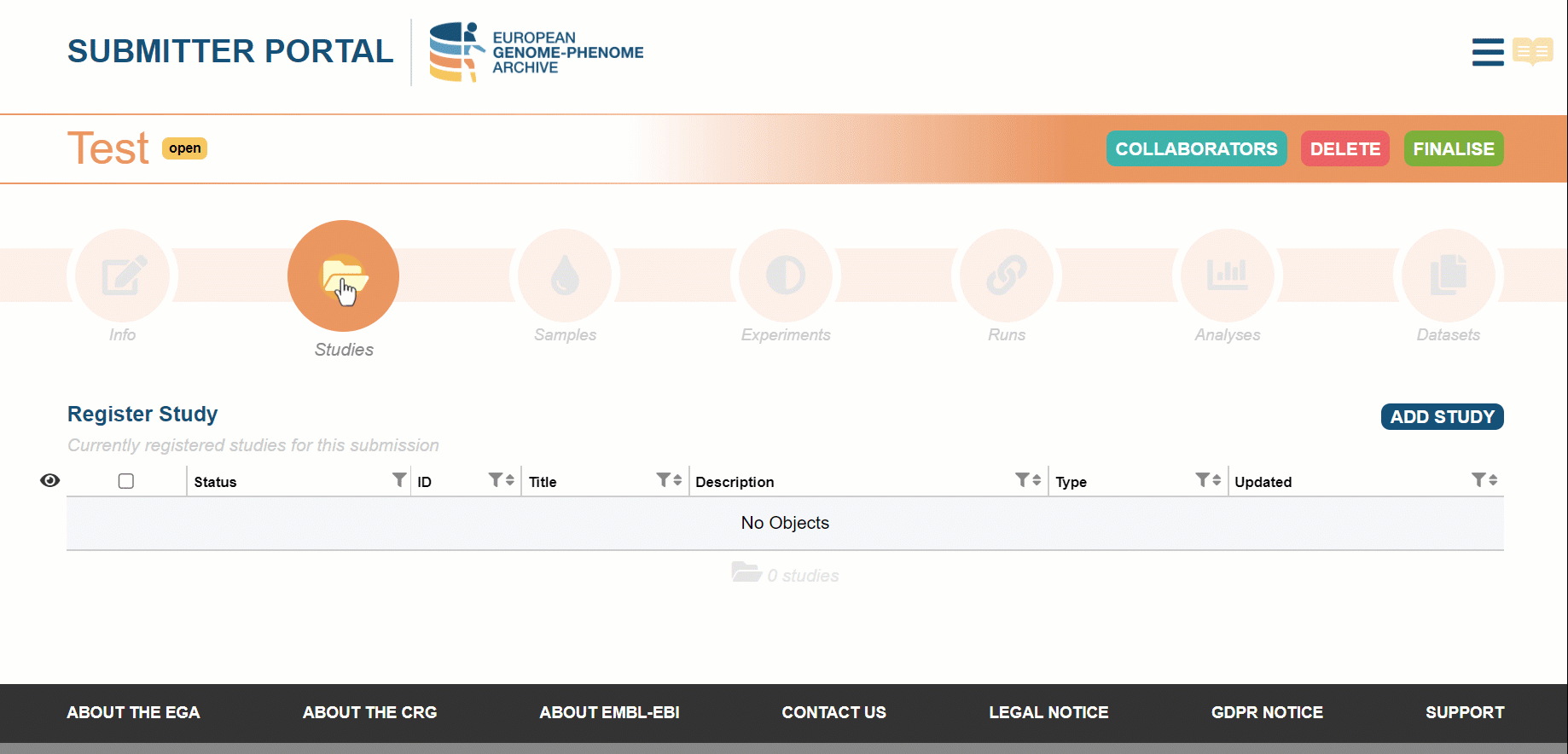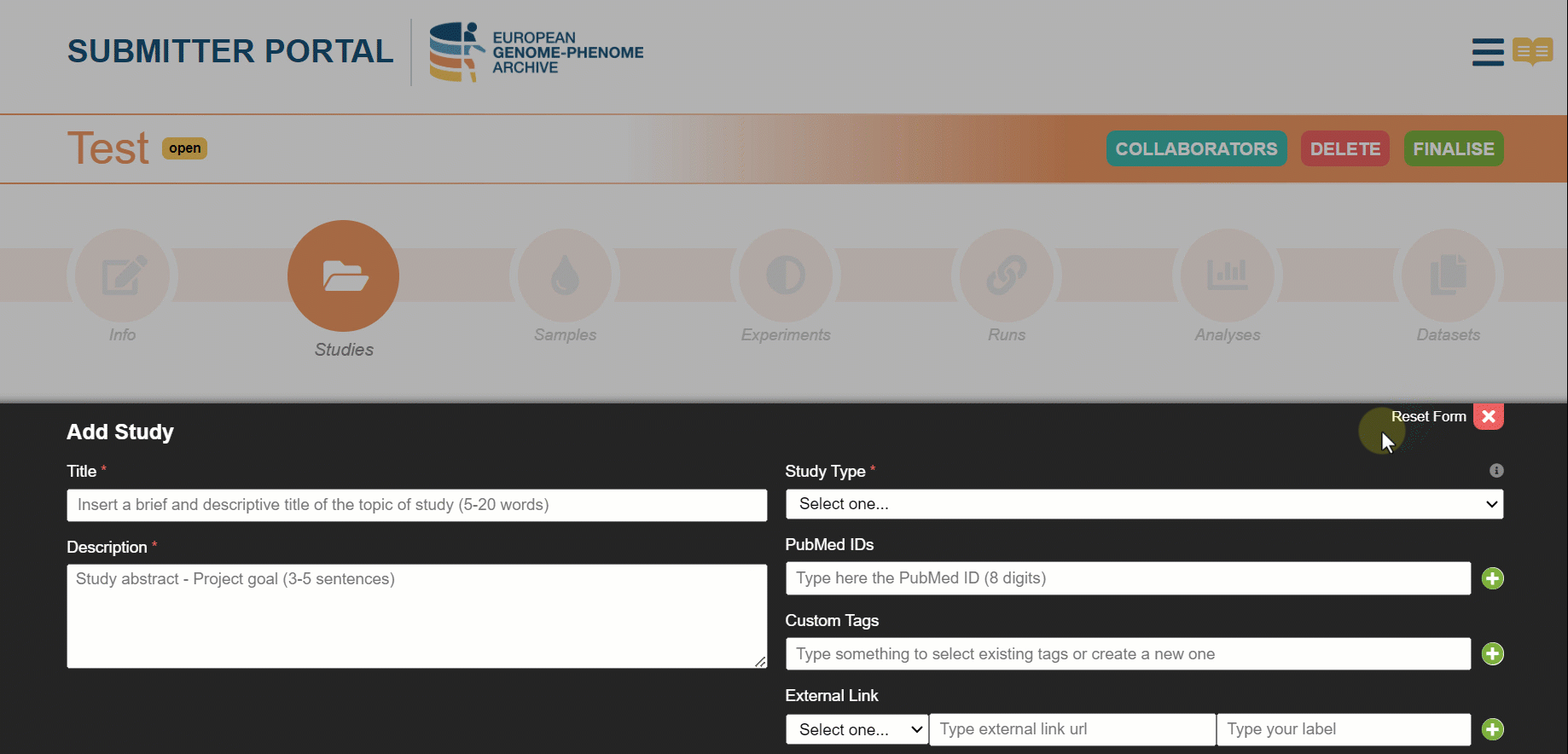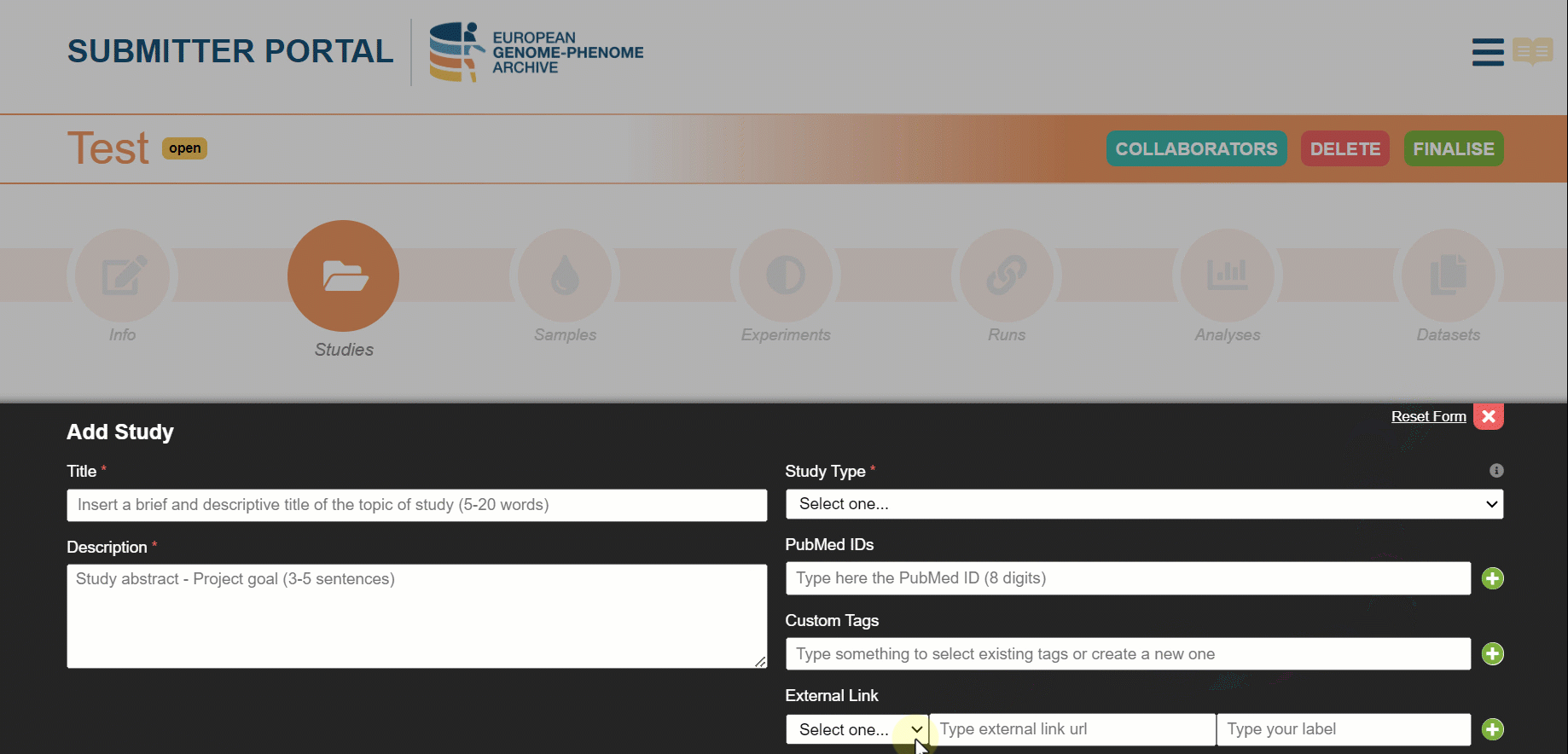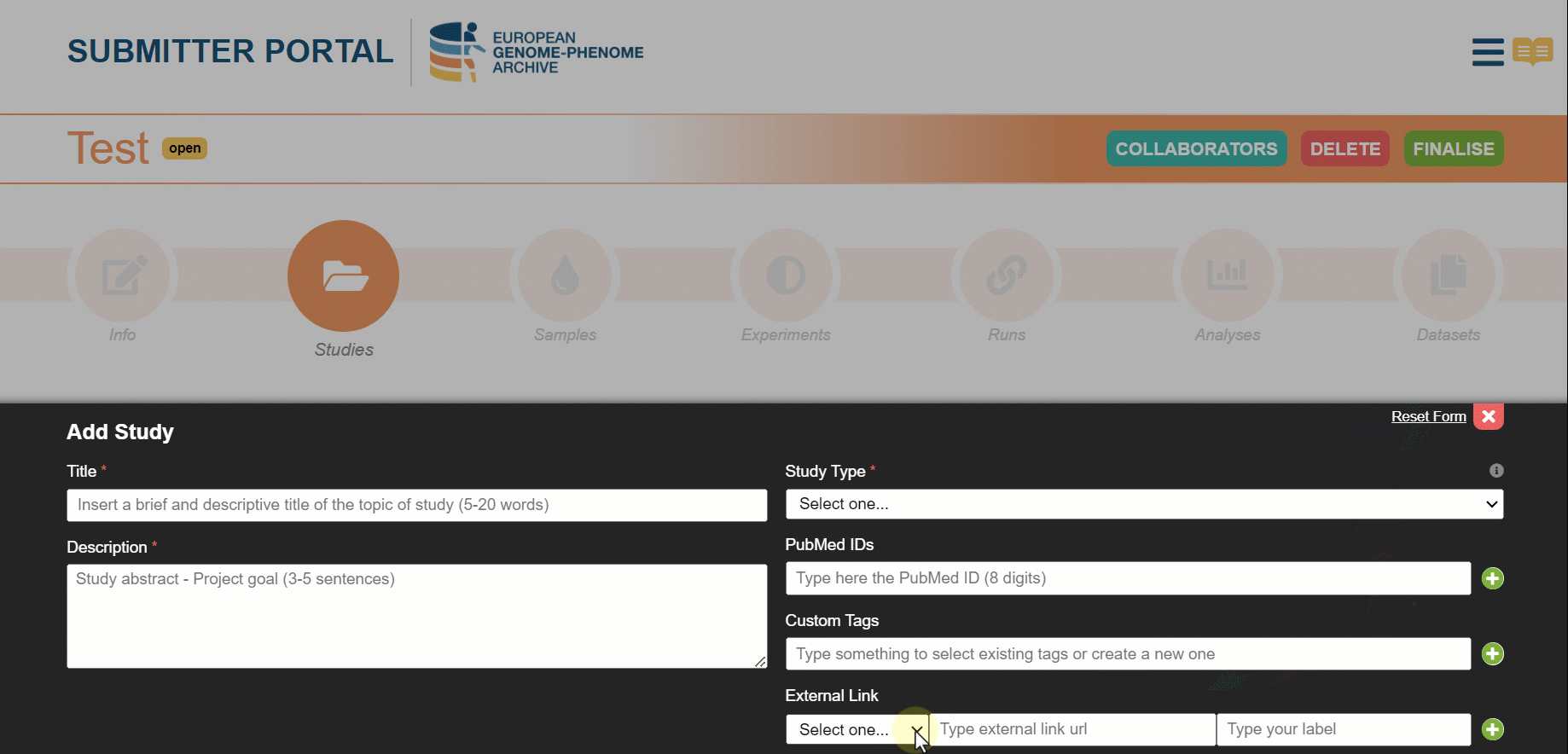
External links are used to connect a study to an external repository. We have implemented an external link to Euro-Bioimaging for a European Project (EuCanImage). Now, we are looking forward to keeping increasing the list of external repositories! Choose the expected release date for your metadata to be searchable on our website
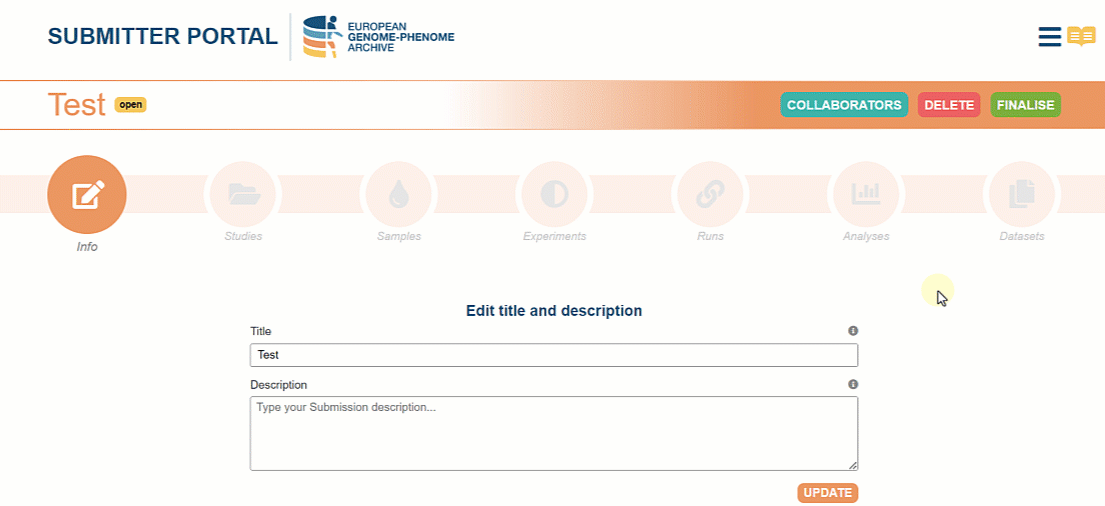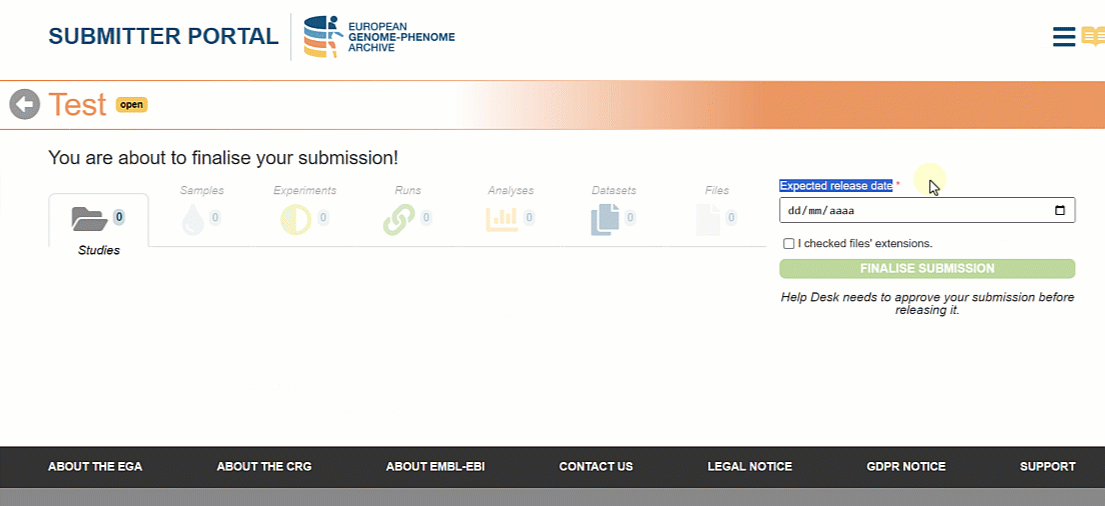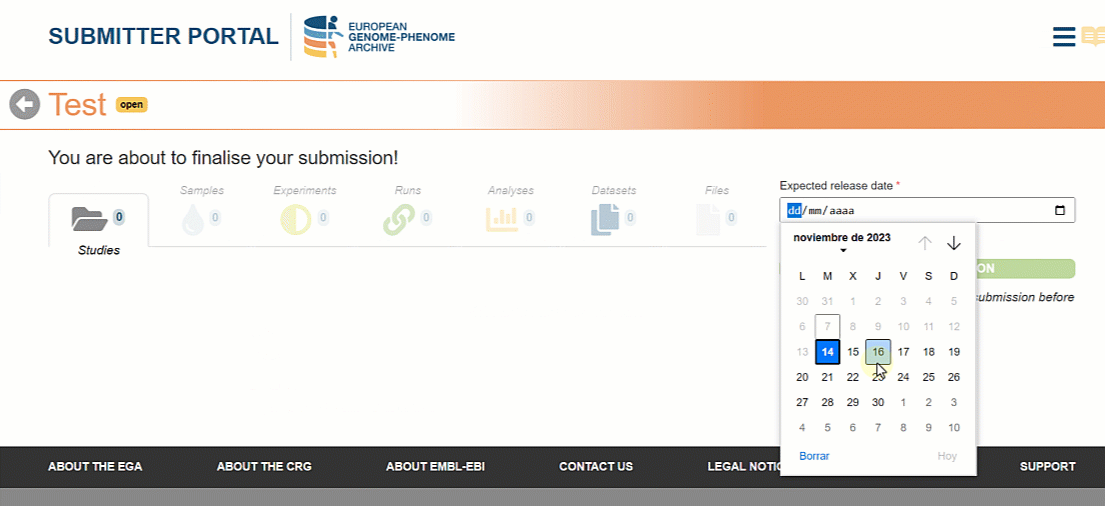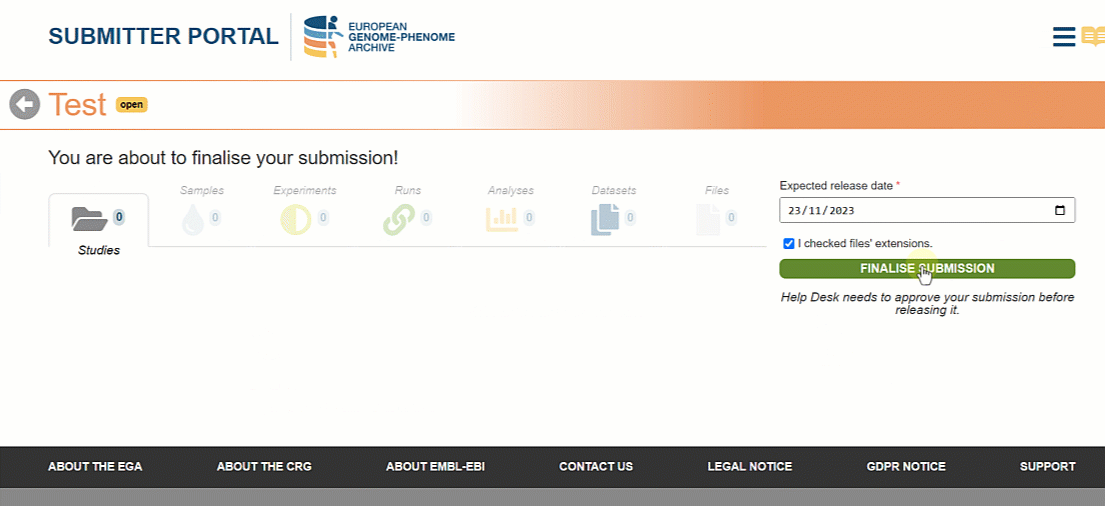
Once all the metadata objects are registered it is time to finalise the submission so that the Helpdesk team can approve it. In the new version of the Submitter Portal users can choose the expected release date for the metadata to be searchable on the EGA website.
Please, note that the selected date will have a minimum of one week margin from the day of finalisation. This time is necessary for your files to be archived and for the submission to be validated by the Helpdesk Team.
Are you looking for the Array submission within the Submitter Portal?
Our Submitter Portal does not support array submissions. The submission of Array-based metadata must be done using the EGA programmatic submission. You will find our Array-based format template and all the necessary information on our website, and you can find all the steps in our submission quickguide!
More information about the submission process at the EGA
Our Team has developed an extended documentation related to metadata submission. A good first step is to take a closer look at the Submission FAQ. To have a general overview of all the metadata submission process we recommend taking a glimpse of our take-the-tour or watching the tutorial available on our YouTube Channel.
Don't miss our section on EGA Schemas, to learn more about how the EGA is built. It can be useful to fully ensure that your submissions comply with the EGA's standards and contribute to a valuable and accessible genomic resource.




